
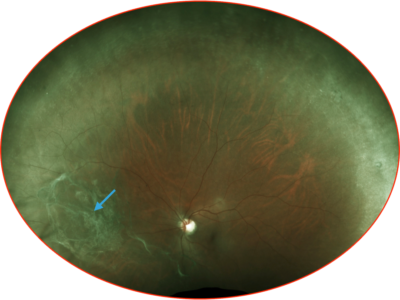
Syndrome, nerve paresis, autoimmune neuropathy Ocular: Uveitis, iritis and other ocular inflammatory toxicities can occur. Pericarditis, vasculitis Nervous System: Meningitis, encephalitis, myelitis and demyelination, myasthenic syndrome/myasthenia gravis (including exacerbation), Guillain-Barré Severe or fatal cases have been reported for some of these adverse reactions. Reported with the use of other anti–PD-1/PD-L1 treatments.

It led to permanent discontinuation of KEYTRUDA in <0.1% (1) and withholding in 0.5% (14) of patients.

Hypothyroidism occurred in 8% (237/2799) of patients receiving KEYTRUDA, including Grade 3 (0.1%) and Grade 2 (6.2%). It led to permanent discontinuation of KEYTRUDA in <0.1% (2) and withholding in 0.3% (7) of patients.
Consider monitoring more frequently as compared to when the drugs are administered as single agents. Monitor liver enzymes before initiation of and periodically throughout treatment.
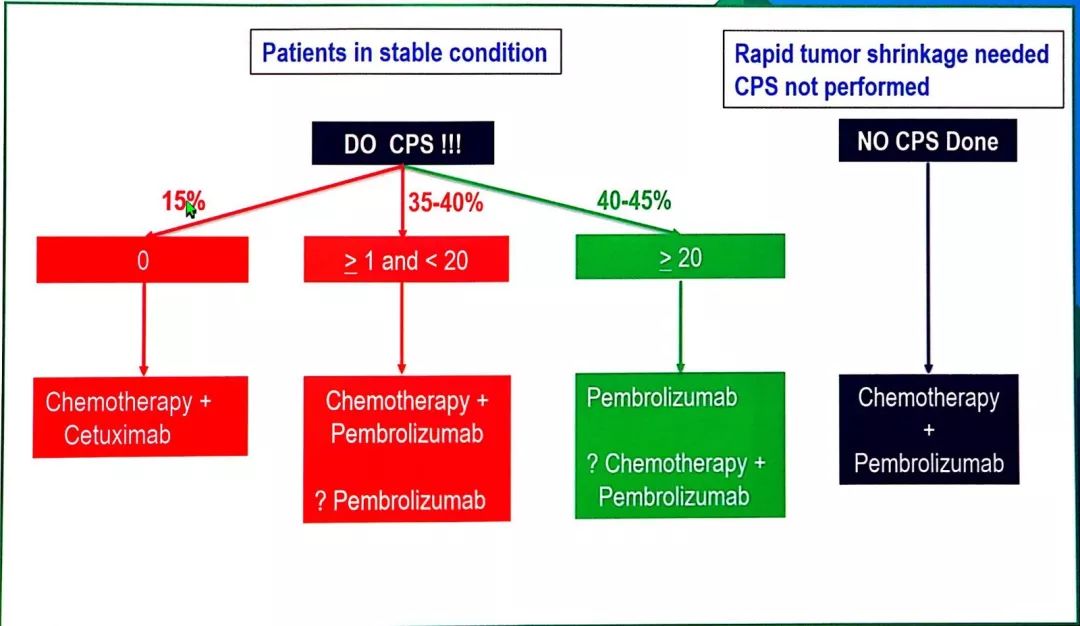
KEYTRUDA in combination with axitinib can cause hepatic toxicity.Metastatic or Unresectable, Recurrent Head and Neck Squamous Cell Carcinoma (HNSCC) Patients with epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genomic tumor aberrations should have disease progression on FDA-approved therapy for these aberrations prior to receiving KEYTRUDA. KEYTRUDA, as a single agent, is indicated for the treatment of patients with metastatic non–small cell lung cancer (NSCLC) whose tumors express programmed death ligand 1 (PD-L1) as determined by an FDA-approved test, with disease progression on or after platinum-containing chemotherapy. stage III where patients are not candidates for surgical resection or definitive chemoradiation, or.KEYTRUDA, as a single agent, is indicated for the first-line treatment of patients with non–small cell lung cancer (NSCLC) expressing programmed death ligand 1 (PD-L1) as determined by an FDA-approved test, with no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genomic tumor aberrations, and is: KEYTRUDA, in combination with carboplatin and either paclitaxel or paclitaxel protein‑bound, is indicated for the first‑line treatment of patients with metastatic squamous non–small cell lung cancer (NSCLC). KEYTRUDA, in combination with pemetrexed and platinum chemotherapy, is indicated for the first-line treatment of patients with metastatic nonsquamous non–small cell lung cancer (NSCLC), with no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genomic tumor aberrations.


 0 kommentar(er)
0 kommentar(er)
